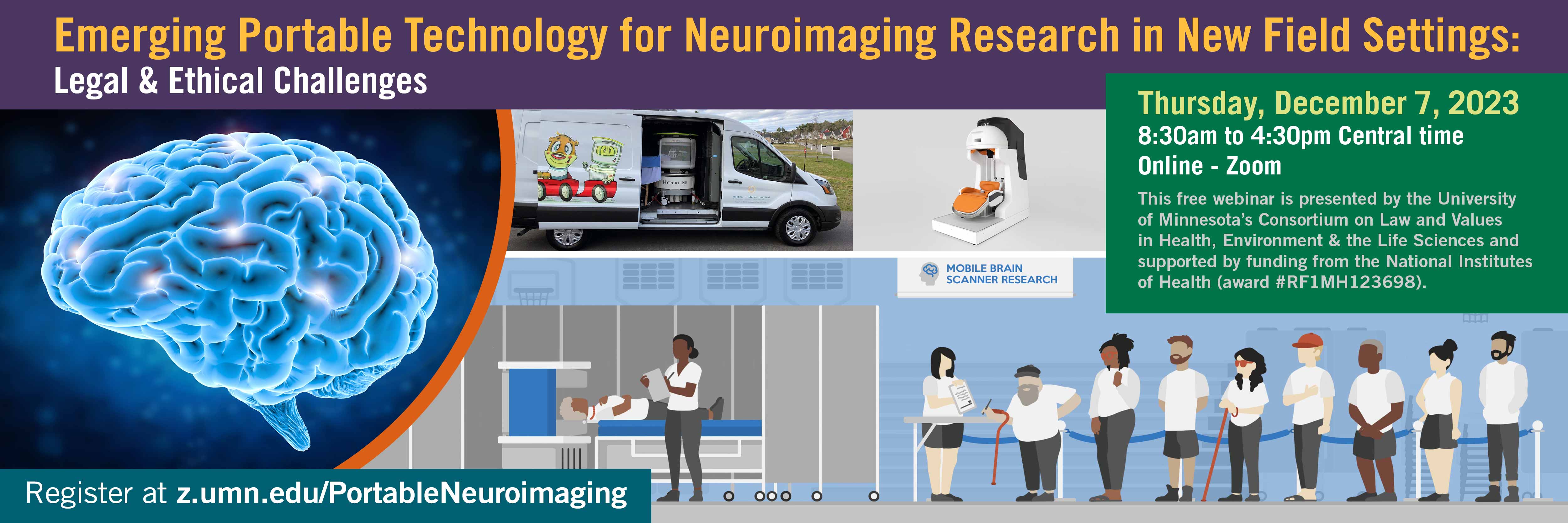
FEATURED EVENTS & RESOURCES
LATEST NEWS & PUBLICATIONS

Congratulations to Rahel Ghebre, MD, with the Masonic Cancer Center (MCC) on being named the University of Minnesota Medical School’s new Associate Dean for Faculty Affairs. Since joining the University in 2005, Dr. Ghebre has been a leader in gynecologic oncology, global health, medical education, and health disparities. Her commitment to improving global health has led to the development of oncology programs in Ethiopia and Rwanda.… Read More

The University of Minnesota Genomics Center (UMGC) recently acquired three additional AVITI sequencers. UMGC now enjoys the distinction of offering the most AVITI sequencers by any individual center in the U.S. The user-friendly AVITI platform is known for its superior data quality. As a result of the expansion, costs to researchers for sequencing projects have dropped by about 50% and turnaround times have accelerated. UMGC, led by… Read More

Angela Birnbaum, PhD, FAES, has co-authored a research article that surveyed nursing home medical directors about current nursing home care practices for residents who suffer seizures. Published in Epilepsy & Behavior, Overview of Acute Seizure Management in US Nursing Homes suggests a lack of uniformity in the industry when responding to those… Read More

The Postdoctoral Fellowship program through UMN’s Institute on the Environment (IonE) is breaking new ground to advance interdisciplinary research. Since 2021, IonE has implemented a fresh approach to postdoctoral mentoring. The fellowship program is designed as a “cohort” experience that encourages a stronger sense of community and teamwork with researchers working on environmental and sustainability projects… Read More